International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1700
ISSN 2229-5518
ADSORPTION ENERGIES OF MERCURY CONTAINING SPECIES ON THE SURFACE OF CALCIUM OXIDE (0 0 1) PREDICTED BY DENSITY FUNCTIONAL THEORY
Michael Mananghaya
Abstract— In this work, the use of computational chemistry methods for predicting Hg and HgCl2 adsorption on CaO was introduced, which has never been studied before with theoretical calculations. The GGA functionals was used to obtain the optimized geome tries and adsorption energies of proposed periodic and adsorption site structures. Also, the goal of the research is to investigate if GGA functionals gives reliable answers compared to available experimental datas.
Index Terms— Adsorption Energy, Calcium Oxide, Density Functional Theory, Mercurous Hg(I) Species, Mercuric-Hg(II) Species
—————————— ——————————
As flue gases are released from coal fired boilers, elemental mercury-Hg(0) is oxidized into mercurous Hg(I), and mercu- ric-Hg(II) species in the form of HgCl and HgCl2 [1], mostly promoted by chlorine and atomic chlorine, as proposed by Sliger, et al. [2], [3]. However, only the mercuric-Hg (II) form (HgCl2) exists in the flue gases because HgCl is directly oxi- dized to HgCl2. The Environmental Protection Agency (EPA) has set regulations on mercury emission concentrations of 0.1 μg Hg / 1 kg coal / day, and choosing appropriate sorbents to reduce Hg emissions is an essential key in meeting this regula- tion. There are many experimental papers on Hg reduction and various kinds of sorbents being used in coal fired power plants, such as activated carbon, calcium-based sorbents, fly ash and zeolites [4], [5]. Finding and exploring the properties of effective and inexpensive sorbents is the current challenge. Paper Waste Derived Sorbent (PWDS) is a newly developed sorbent and was proved to be very effective in a bench scale Hg control system [6]. The components in PWDS are CaO 23
wt%, Al2O3 SiO2 29 wt%, CaCO3 41 wt%, inert 6 wt% and
Ca(OH)2 1 wt%. Since CaO is one of the primary constituents in this novel sorbent that could be used in coal fired power
plants, the adsorption of Hg and HgCl2 on the CaO surface were investigated so the fundamental interactions between Hg-species and the sorbent could be explored.
It is very difficult to examine the adsorption mechanisms of Hg-containing species on surfaces experimentally [7]. On the other hand, theoretical methods can be employed to investi- gate how Hg-containing species are adsorbed on the sorbent. The increase in computer speed and the accuracy of computa-
————————————————
Michael Mananghaya is a research scholar at De La Salle University, Phil-
ippines. mikemananghaya@gmail.com
tional quantum chemistry methods leads them to be applied to the study of surface chemistry more frequently. Meanwhile, understanding the adsorbents’ surface properties theoretically will help us to choose the best adsorbent for certain adsorb- ates. We studied Hg and HgCl2 adsorption on the CaO (0 0 1) surface by performing Density Functional Theory (DFT) calcu- lations using Generalized Gradient Approximation (GGA/BLYP) functionals on periodic model in this work.
DFT with the DMol3 [8] package in the Accelrys Materials Studio 2001 (Accelrys Inc.) was used for this study. All simula- tions for periodic CaO models used the AE (all electron) method and DNP (double-numeric) basis set. The GGA was combined with the Becke-Lee-Yang-Parr (BLYP) correlation functional [9], [10], [11]. The higher-level calculations of the GGA/BLYP method used the fully optimized geometries to obtain the adsorption energies. Mulliken and Hirshfeld popu- lation analyses were computed, not only to calculate atomic charges for Hg and and HgCl2 adsorption models on CaO pe- riodic slabs, but also to investigate how the mercury- containing species interact with the model CaO surfaces.
Spin unrestricted calculations on the periodic slab were also conducted because spin polarization could have an affect on the adsorption energies. Spin-orbit coupling effects are ne- glected in this scalar relativistic approach based on prior re- search with other mercury calculations [12]. The numerical basis functions of DMol3 are more complete functions than Gaussian functions, and are expected to have small BSSE (the basis set superposition error) contributions while ab initio methods use BSSE calculations to estimate the errors associat-
IJSER © 2013
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1701
ISSN 2229-5518
(a)

(b)
(c)
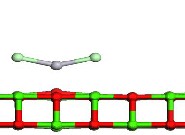
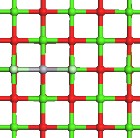
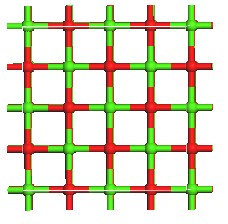
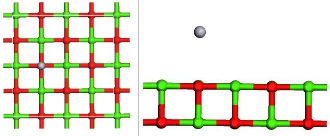
Fig.1. Optimized geometry of Structure of Hg and HgCl2 adsorption on CaO (a) the infinite CaO (0 0 1), (b) Hg consistently adsorbed on an atop site over the oxygen atoms on the surface of the CaO (0 0 1), (c), HgCl2 adsorbed on the CaO (0 0 1) wherein each chlorine atom gravi- tated to calcium sites. Green color depicts calcium atoms; light green is Chlorine, red is Oxygen, and gray is Mercury.
ed with their incomplete basis sets [13]. A small BSSE contri- bution due to the basis sets of DMol3 was verified by calculat- ing water dimer with a larger basis set. The interaction energy was changed by less than 0.5 kcal/mol in these calculations [14], [15]. They, indirectly, proved that further basis set exten- sions have a small BSSE effect on interaction energies.
The surface of CaO (0 0 1) that has been used by other the- oretical researchers [16] was investigated. It should be noted
that the 3×3×2 cluster model had edge effects but 5×5×2 clus- ter models succeeded in size convergence [17]. The 5×5×2 clus- ter models are large enough to handle the adsorbates studied here. In this work, the clean perfect surface was modeled by a
5×5×2 periodic structure. The Hg coverages for the periodic slab is 4.0% since the surface was designed with a 5×5 unit cell. These low coverages were used to approximate pure component adsorption on fresh adsorbates, which would overestimate the results for aged systems. Two surface layers of metal-oxide were employed to investigate interactions be- tween the adsorbate and surface [18]. For some calculations, all CaO (0 0 1) structures were fixed with a bond length of 2.4
Å between every distance for the Ca–O pairs for rapid investi- gation of mercury adsorption. This bond length is the default for the CaO (0 0 1) cleaved surface in DMol3. The periodic sys- tem studied has a vacuum thickness of 10 Å. This distance was chosen to eliminate spurious interactions between the adsorb- ate and the periodic image of the bottom layer of the surface, which would appear on the top of the 5×5 unit cell. Also, the k-point was set to the 2×2×1 mesh by Monkhorst-Pack scheme [19] in this calculation on the periodic slab.
Hg consistently adsorbed on an atop site over the oxygen at-
oms on the surfaces, and each chlorine atom gravitated to cal- cium sites according to the optimized geometries, as shown in Figure 1. Bond lengths for each species indicate which species are more strongly adsorbed to the surface. The distances be- tween mercury and oxygen on the 5×5×2 surface are 3.535 Å
for Hg(0) and 2.344 Å for Hg(+2) at the present calculation levels. The chlorine atom has a bond length of 3.258 Å to the surface with calcium. In Figure 1.c the movement of the oxy- gen out of the flat surface makes it more favorable for strong interactions with mercury so that the Hg is much closer to the surface at 2.344 Å. the relaxation effect is not essential for the Hg adsorption model, the relaxation effect plays an important role in the HgCl2 adsorption model to move the adsorption energy into the chemisorption range as discussed in the next section.
The adsorption energies of mercury-containing species on the modeled structures, investigated with GGA/BLYP methods were calculated through the following equation:
ΔEads = EHg-species+surface – EHg-species – Esurface (1)
where ΔEads is the adsorption energy, EHg-species+surface represents the energy of the adsorbate species–surface, EHg-species is the energy of the gas phase mercury–containing species, and Esur- face is the energy of the isolated periodic model, in kcal/mol and the results indicate a –4.84 kcal/mol for Hg(0) and –30.28 kcal/mol for HgCl2. These show the trends as the adsorption strength is changed for the different oxidation states. Metallic
IJSER © 2013
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1702
ISSN 2229-5518
mercury, which is not reactive, has a weak adsorption on the
5×5×2 periodic slab. The adsorption of elemental mercury on
the periodic structure is attributed to be physical adsorption
according to the fact that the adsorption energy of Hg(0) from the GGA/BLYP calculations falls into the physical adsorption range of about 6 kcal/mol [20]. The oxidized mercury in the form of HgCl2 over metallic Hg has a stronger adsorption en- ergy value on the 5×5×2 periodic slab. These results suggest that CaO will be a stronger adsorbent for the reactive oxidized mercury species. The adsorption energies of HgCl2 on the CaO surfaces is chemisorption [17]. The chlorine atoms released from HCl or Cl2 in the coal combustion flue gas system greatly enhance the adsorption capability of the CaO according to these predicted results. The fact that fluoro-, bromo-, and chlo- ro-carbons are destructively adsorbed on the CaO surfaces and that calcium oxides can be effective in removing halogen- ated carbons as shown by Decker, et al. [21] is consistent with the results.
Charge analyses results depend on the methods used and show how electron density is partitioned. In order to under- stand the trends in atomic charge transfer, atomic charges with two different methods including the Mulliken and the Hirshfeld population analyses are compared. The Mulliken method is the most commonly used population method and the Hirshfeld population analysis is based on the deformation density on the free atom electron density. The atomic charges for a fixed clean periodic CaO slab and CaO slabs reacted with Hg and HgCl2 were used to obtain the final Mulliken and Hirshfeld charges. The Mulliken charges of the mercury atoms in Hg and HgCl2 on the surfaces are changed by -0.041 and -
0.069, respectively and the chlorine is negatively charged to -
0.145. On the CaO surface, the final atomic charges of the
nearby calcium atoms were almost the same after CaO–Hg adsorption while all charges on nearby oxygen atoms were changed positively after CaO–mercury species were formed. The adsorption of CaO–HgCl2 receives more charge transfer
between HgCl2 and the CaO surface compared with the mer- cury adsorptions. This is intuitive because the adsorption of HgCl2 is stronger because bonding occurs, as described earlier. The trends in the Hirshfeld charges are similar to the Mulliken charge analysis; the Hirshfeld charges of the nearby calcium atoms were changed on the average by -0.03 for Hg adsorption and -0.03, -0.03, -0.07 and -0.07 for HgCl2. This is consistent with the Mulliken charge analysis as the same calcium atoms of the surface interact with mercury adsorbates. Again, when the mercury is oxidized, mercury is charged more negatively. This result is in good agreement with the fact that adsorption becomes stronger when a larger charge transfers occur. The comparison of results of adsorption energies is in accordance with the results of the charge transfer calculations.
The adsorption energies of Hg, HgCl and HgCl2 adsorption on
CaO surfaces were calculated by GGA/BLYP methods. Ele-
mental mercury adsorption on the CaO (0 0 1) surface is phy- sisorption in nature. Additionally, HgCl2 on the calcium oxide surface are at chemisorption. Stronger adsorption occurs when mercury is oxidized. The oxidized mercury has an active in- teraction with the surface according to the Mulliken and Hirshfeld charge analyses. This research paper is the first in a series of investigating the mechanism of mercury adsorption on PWDS. Since flue gases are generally released at high tem- peratures, the CaO component of PWDS might not be a very strong adsorbant itself to remove mercury. Future work may examine more chemical arrangements that include CaO com- ponents as a part of a more complex sorbent environment.
This work was supported by the Department of Science and Tech- nology, Philippine Council for Industry, Energy and Emerging Technology Research and Development (PCIEERD) formerly Phil- ippine Council for Advanced Science and Technology Research and Development (DOST-PCASTRD) for the acquisition of the Dmol3 v6.0 software. Also, this was supported in part by the Science Education Institute (DOST-SEI) and the Physics and Chemical En- gineering Department of De La Salle University-Manila.
[1] S. Niksa and N. Fujiwara, “Fundamentals of Mercury Oxidation in Flue Gas ,“
Environ. Sci. Technol. vol. 31, pp. 3701-3706, 2001.
[2] K. C. Galbreath, “Mercury speciation in coal combustion and gasification flue gases,” Environ. Sci. Technol. vol. 30, pp. 2421-2426, 1996.
[3] R. N. Sliger and N. M. Marinov, “A Global Kinetic Mechanism for the Predic- tion of Hg Oxidation by a Chlorine Specie ,” Fuel Process. Technol. vol. 65, pp. 423-438, 2000.
[4] Y. Otani, C. Kanaoka, I. Uchijima and H. Nishino, ” Environmental Aspects of
Trace Elements in Coal ,” Environ. Sci. Technol. vol. 22, pp. 708-711, 1988.
[5] Y. Otani, C. Usui, S. Matsui and H. Emi, ”Mercury from Gold and Silver Min- ing: A Chemical Time Bomb?,” Environ. Sci. Technol. vol. 20, pp. 735- 738,
1986.
[6] J. O. L. Wendt, S. J. Lee and J. Biermann, 6th International Symposium on Coal
Combustion (ISCC) Wuha, P. R. China, Dec. 1-4, 2007.
[7] C. Noguera,”Electron redistribution in low-dimensional oxide structures,”
Surf. Rev. Lett. vol. 8, pp. 121, 2001.
[8] B. Delley, “An All-Electron Numerical Method for Solving the Local Density Functional for Polyatomic Molecules,” Journal of Chemical Physics, vol. 92, pp. 508-517, 1990.
[9] C. T. Lee, W. T. Yang and R. G. Parr,” Theoretical and Experimental studies of N-(6-methylpyridin-2-yl-carbamothioyl)biphenyl-4-carbox amide,” Phys. Rev. B. vol. 37, pp.785-789, 1988.
IJSER © 2013
International Journal of Scientific & Engineering Research, Volume 4, Issue 4, April-2013 1703
ISSN 2229-5518
[10] A. D. Becke, “Nuclear Magnetic Resonance Shielding Tensors ,” J. Am.
Chem. Soc. vol. 195(35-PHYS), 1988.
[11] A. D. Becke, “The X3LYP extended density functional for accurate descrip- tions of nonbond interactions, spin states, and thermochemical properties,” Phys. Rev. A. vol. 38, pp. 3098-3100, 1988.
[12] S. D. Williams and E. E. Edwards, “Scalar Relativistic Study of the Structure of Rhodium Acetate,” Int. J. Molecular Sciences vol. 5, pp. 67-74, 2004.
[13] A. Simperler, P. A. Bonnet, W. Jones and W. D. S. Motherwell, “Correlation of melting points of inositols with hydrogen bonding patterns,”Cryst. Eng. Comm. vol. 8, pp. 589-600, 2006.
[14] N. Govind, K. Reindel and G. Fitzgerald, “Zeolite-Catalyzed Hydrocarbon
Formation from Methanol,” Int. J. Mol. Sci. vol. 3, pp. 423-434, 2002.
[15] J. G. Andzelm, Niranzan, G. Fitzgerald and A. Maiti, “Reactivity of a Phos- pholipid Monolayer Model under Periodic Boundary Conditions,” Int. J. Quant. Chem. vol. 91, pp. 467-473, 2003.
[16] G. Pacchioni and F. Illas, ”Calcium Adsorption on MgO(100): Energetics, Structure ,” J. Amer. Chem. Soc. vol. 116, pp. 10152-10158, 1994.
[17] X. Li, "A Density Functional Theory Study of Mercury Adsorption on Paper
Waste Derived Sorbents." University of Arizona, 2006.
[18] E. J. Karlsen and L. G. M. Pettersson, “Liquid Water Structure from X-ray Spectroscopy and Simulations of water and hydroxyl at metal surfaces,” J. Phys. Chem. B. vol. 107, pp. 7795-7802, 2003.
[19] H. J Monkhorst and J. D. Pack “Special points for Brillouin-zone integra-
tions,” Phys. Rev. B. vol. 13 pp. 5188-5192, 1976.
[20] A. W. Adamson and A. P. Gast, Physical Chemistry of Surfaces 6th ed.; Wiley: New York, 1997.
[21] S. P. Decker, A. Khaleel and K. J. Klabunde, “Synthesis of magnetite– mesoporous silica composites as adsorbents for desulfurization from natural gas,” Environmental Science Technology vol. 36, no. 4, pp. 762-2, 2002.
IJSER © 2013