International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2963
ISSN 2229-5518
A Statistical Learning tool for identifying
Elemental ratio in Cerebrospinal fluid
Siddaraju K. 1, Dr. Sanjay Pande 2
1: Research Scholar, CMJ. University & Asst. Professor of Computer Science, .Maharani’s Science College for Women, Mysore-570005, Karnataka, India
2: Research Guide, Principal, SITAR, Belakere, Channapatna-562160, Karnataka, India
—————————— ——————————
In a medical diagnosis problem, what is needed is a set of examples that are representative of all the variations of the disease. The examples need to be selected very carefully if the system is to perform reliably and efficiently. The fact that there is no need to provide a specific algorithm on how to identify the disease, presents a major advantage over the application of machine learning methods to this type of problems. In other problems, such as sensor monitoring during surgery, the ability of these methods to provide sensor fusion, also presents another reason for their use.
However, development of machine learning systems for medical decision making problems is not a trivial task. Difficulties include the acquisition, collection and organization of the data that will be used for training the system. This becomes a major problem especially when the system requires large data sets over long periods of time, which in most cases is not available due to the lack of an efficient recording system. In medical imaging tasks, for example, there is still no scheduled procedure for digitising scans, such as scans printed on film (X-rays) or specimens for microscope screening. Another difficulty arises when trying to automate some processes as not all of them can be automated due to ethical and safety issues. Deciding what could and needs to be automated directly influences the design and implementation of the learning system.
Alzheimer’s disease (AD) is a progressive neuro- degenerative disease associated with age (4% above 65 years) and more prevalent in people aged above 80 years. Another prominent feature of AD is neuronal cell death (Morrison and Hof 1997; Su et al 1997). Clinical hallmarks are progressive deterioration of memory and cognition . AD has a devastating effect on cognitive behavior and ability to perform daily tasks, which imposes burdens on caregivers. But no specific diagnostic parameter has been established till now. This needs concern, as early diagnosis will help in delaying the progression of the disease and if possible cure. Attempts have been earlier made to use cerebrospinal fluid (CSF) as a diagnostic tool by analyzing tau and amyloid beta protein (aβ) etc. Trace elements have been implicated in AD pathology. Limited reports are available on trace elements distribution in CSF in AD (Basun et al 1991) but no studies have been made to correlate inter-elemental relationship. In the present investigation, we studied trace element interrelations in CSF in normal and AD patients. To the best of our knowledge, this is the first report presenting a comprehensive data on trace element concentrations, mole percentage and inter-elemental relationships in CSF.
Eleven normal and nine AD CSF samples were used in the present study. CSF samples were collected after taking the written consent of the patient. Patients with AD met the standard diagnostic criteria of NINCDS-ADRDA (McKhann et al 1984). Individuals with no known neurological problems served as normal. CSF samples were
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2964
ISSN 2229-5518
obtained through Human Brain Tissue Repository (HBTR), Dept. of Neuropathology, National Institute of Mental Health and Neuroscience, Bangalore, India. All the precautions were taken to eliminate metal contamination while collecting and during storage of the samples in accordance with The National Committee for Clinical Laboratory Standards, USA, criteria (1997). All dilutions were made with ultra pure water (18-mega ohms resistance) in dust free room. Element analysis was done by Inductively Coupled Plasma - Atomic Emission Spectroscopy (ICP-AES), either by sequential or simultaneous mode depending on the elements to be analyzed. The optimization of ICP-AES was evaluated by line selection and detection limits for each element (Rajan et al 1997). The validation of the analysis was tested by analyzing CSF matrix match multi-element synthetic standard and certified standard reference material obtained from National Bureau of Standards, USA (Rajan et al, 1997).
Generally, for a given learning task, with a given finite amount of training data, the best generalisation performance will be achieved if the right balance is reached between the accuracy attained on the particular training set, and the ability of the machine to learn any training set without error, that is, its capacity. A trained machine with very large capacity will overfit on the training data and will be able to identify only previously seen examples, while a machine with very small capacity will not be able to identify even previously seen data, i.e. learning of the training data will be incomplete. Neither can generalise well and therefore, the relation between the capacity and the performance of a learning machine must be controlled to achieve the right balance.
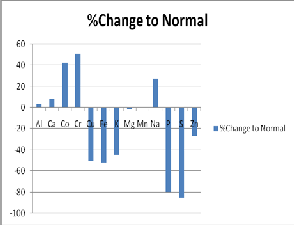
The data was expressed as micromoles/ml with mean, range of values, percentage change, standard deviation and p-values (test of significance) (Table 1).
The mole percentage (Elemental concentration in mole% = Elemental concentration (µ mole/g) x 100/Total elemental concentration (µmole/g) of analyzed elements in each region) were calculated for the analyzed elements (Table 1).
Mole percentage calculations are essential to understand the relative distribution of each element in relation to other elements in biological matrix and this will also help to normalize the data of different samples to arrive at a clear inter-element relationships.
Element to element ratios were calculated based on mole percentage to arrive at a possible elemental inter- relationships between normal and AD CSF.
The total number of charge in terms of single (+) (Na, K), double (++) (Mg. Cu, Zn, Ca), or triple (+++) (Al, Fe) charged ions distribution was calculated using the formulae:
Sum of concentration of particular element in micro mole per gram x Z x Avogadro number x 10-6, where Z is 1, 2, 3 for single, Double, triple charge calculation respectively.
The comparative assessment of trace elements between normal and AD CSF indicated the following trends (Table
1).
There was no significant change in the levels of Al, Ca, Mg, and Mn between normal and AD CSF. K, P and S were significantly (P<0.0001) decreased in AD CSF when compared to normal while Na level was significantly (p<0.0001) increased in AD CSF.
The elements Co and Cr were elevated in AD CSF, while Cu and Fe levels were decreased in AD CSF. These comparisons were only at the level of p=0.04. Similarly, the Zn level was moderately decreased in AD CSF (p=0.04).
Essential trace elements are required at very low concentration for the proper functioning of human biological system but at higher concentration they are toxic. The non-essential trace elements like Al also accumulates in the system and is likely to play a role in pathology of certain neuro-degenerative diseases (McDermott et al 1979).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2965
ISSN 2229-5518
Even other essential trace elements like Fe, Cu and Zn also have been implicated in the pathology of neurological diseases (Smith et al 1997; Cornett et al 1998).
Substantial information is available on trace metals distribution in brains of normal aging (Markesbery et al
1984) and neurodegenerative diseases like Alzheimer’s, Parkinson’s and Huntington disease (Dexter et al 1991). However, limited data is available on trace elements concentration in CSF. An increase in Cu, while Ca and Cd decreased in CSF of patients diagnosed as suffering from Dementia of Alzheimer’s type. In our findings, we observed that Cu was decreased in AD CSF, while Ca did not show any change.
Hershey et al (1983,1984) reported an increase in Si although there was no significant change in Al, Mn, As, Pb in CSF of AD. Our findings regarding Al and Mn were in agreement with the above findings. A report of decrease in Zn, while no change in Cu and Mn in AD CSF was observed. This current study similarly found a decrease in Zn and also in Cu levels while the Ca level remained unchanged. A significant decrease in AD CSF for both Ca and P. We failed to detect a change in Ca levels in AD CSF.
Furthermore, there is no global database for CSF trace elements even for normal individuals except only one detailed report on Italian subjects (Sabbioni et al., 1992). Our present findings on Fe and Cu were within the range reported by the above group, but Cr, Co and Zn were found to be very high in normal CSF of Indian population compared to Italian subjects – suggesting such variation could be influenced by national ethnic origin. However, since this group did not perform any analyses for Al, Na, S, K, Mg and P, we can not compare our data in respect of these elements.
The comparative analysis of the distribution in the brain of trace elements( Deibel et al 1996) and CSF of AD patients and normal individuals indicates the following situation prevails. The levels of K and P decreased in both brain and CSF of AD compared to their respective normal samples.
This shows direct relation.
Fe and S were increased in AD brain, while decreased in
AD CSF compared to their respective normal. This shows
inverse relation.
Al was significantly elevated in AD brain over normal
brain, but no significant between AD and normal CSF.
Na was significantly decreased in AD brain over normal,
while in moderate change was observed in AD and normal
CSF.
The element to element mole percentage ratios Al/Fe and Fe/S were decreased in AD brain over normal, while in AD CSF these ratios were increased. This shows inverse relation.
Al/Zn, Fe/Cu, Zn/Cu were elevated in AD brain and AD
CSF compared to their respective normal. This shows direct relation.
The ratios Al/Ca and Al/Mg were elevated in AD brain, while in AD CSF these two ratios did not change.
The total percentage elemental charge distribution namely monovalent and divalent were decreased in AD brain while trivalent charge was increased compared to normal brain. In AD CSF, there was marginal change in the charge distribution pattern compared to normal.
.
Al, Mg, Mn and Ca levels did not show change between normal and AD. However, elements namely K, P, and S were significantly decreased in AD CSF over normal, while Na level was significantly increased in AD CSF. Further, Co and Cr were elevated in AD CSF, while Cu and Fe levels were decreased in AD CSF. Mole percentage ratio of selected elements showed a definite increasing trend in AD CSF over normal. The comparative assessment of data between brain and CSF showed a definite inter-relations. The comparative assessment showed a definite trace elemental inter-relationships (direct or inverse) between brain and CSF of AD. Further, this information may possibly aid in understanding AD progression. This information provides a clue in understanding the role of trace elemental homeostasis in neurodegeneration.
[1] American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, American Psychiatric Association, Washington D.C.
[2] Basun H, Forssell LG, Wetterberg L et al. (1991) Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer’s disease. J. Neural Transm. Park. Dement. Sect. 3(4), 231-258.
[3] Cornett CR, Markesbery WR and Ehmann WD (1998) Imbalances of trace elements related to oxidative damage in Alzheimer’s disease brain. Neurotoxicol. 19 (3), 339-345.
[4] Deibel MA, Ehmann WD and Markesbery WR (1996) Copper, iron and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: possible relation to oxidative stress. J. Neurol. Sci. 143, 137-142.
[5] Dexter DT, Carayon A, Javoy-Agid F et al. (1991) Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114, 1953-
1975.
[6] Hershey CO, Hershey LA, Varnes AW et al. (1983) Cerebrospinal fluid trace element content in dementia: Clinical, radiological and pathologic correlation. Neurology 33 (10), 1350-1353.
[7] Hershey LA, Hershey CO, Varnes AW. (1984) CSF Silicon in dementia: A prospective study. Neurology 34, 1197-1201
[8] Markesbery WR, Ehmann WD, Alauddin M et al. (1984) Brain trace element concentrations in aging. Neurbiol. Aging. 5, 19-28.
[9] Mc Dermott JR, Smith AI, Iqbal K et al. (1979) Brain aluminum in aging and Alzheimer disease. Neurology 29, 809-814.
[10] Mc Khann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984). Clinical diagonis of Alzheimer’s disease: Report of the NINCDS- ADRDA Work Group under the auspices of Department of Health and
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2966
ISSN 2229-5518
Human Services Task Force on Alzheimer’s disease. Neurology 34, 939-
944.
[11] Morrison JH and Hof PR (1997) Life and death of neurons in the aging brain. Science 278, 412-419.
[12] The National Committee for Clinical Laboratory Standards (NCCLS,1997): Control of pre-analytical variation in trace element determinations, Approved guide lines. Vol. 17(13) pp 1-30. 940 West Valley road, suite 1400, Wayne, Pennsylvania 19087, USA.
[13] Rajan MT, Jagannatha Rao KS, Mamatha BM et al. (1997) Quantification of trace elements in normal human brain by inductively coupled plasma atomic emission spectrometry, J Neurol. Sci. 146, 153-166.
[14] Sabbioni E, Minoia C, Pietra R et al. (1992) Trace element reference values in tissues from inhabitants of the European Community. II. Examples of strategy adopted and trace element analysis of blood, lymph nodes and cerebrospinal fluid of Italian subjects. Sci. Total Environ. 120, 39-62.
[15] Smith MA, Harris PLR, Sayre LM et al. (1997) Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA. 94, 9866-9868.
[16] Su JH, Deng G and Cotman CW (1997) Bax protein expression is increased in Alzheimer’s brain: Correlation with DNA damage, Bcl-2 expression and brain pathology. J. Neuropathol. Ex. Neurol. 56, 86-93.
TABLE 1. PERCENTAGE CHANGE OF CHEMICAL TRACE
ELEMENTS.

Statistically significant *- P<0.0001; **- P=0.01;
***-P=0.04.
TABLE 2. MOLE PERCENTAGE RATIO IN CSF OF AD AND NORMAL
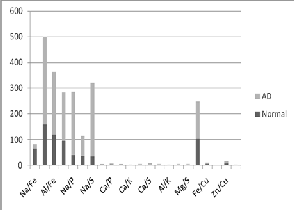
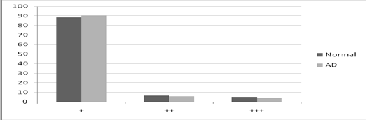
TABLE 3. STATISTICAL ELEMENTAL CHARGE DISTRIBUTION IN NORMAL AND AD CSF (+:MONOVALENT; ++: DIVALENT; +++: TRIVALENT IONS)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Vo lume 4, Issue 6, June-2013
ISSN 2229-5518
2967
IJSER lb)2013
International Journal of Scientific & Engineering Research, Vo lume 4, Issue 6, June-2013
ISSN 2229-5518
2968
IJSER lb)2013
International Journal of Scientific & Engineering Research, Vo lume 4, Issue 6, June-2013
ISSN 2229-5518
2969
IJSER lb)2013